TECHNICAL INFORMATION
HEAT TREATMENTS APPLIED TO STEELS
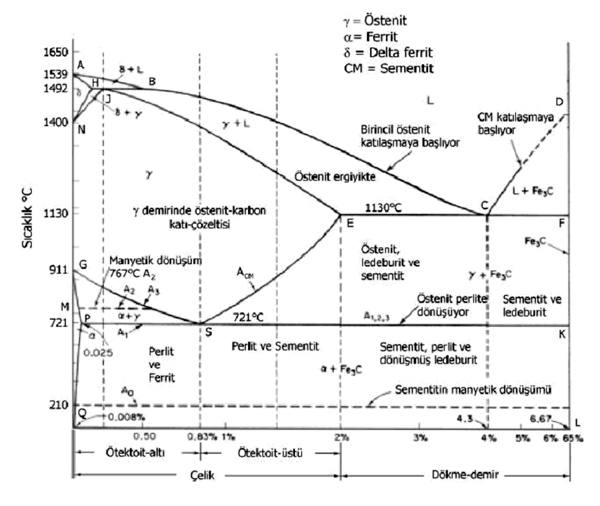
IRON CARBON BALANCE DIAGRAM
 DESCRIPTION OF HEAT TREATMENT
DESCRIPTION OF HEAT TREATMENT
Heat treatment is generally described as controlled heating and cooling treatments applied in solid phase to metals or alloys to provide desired peculiarities.
All fundamental heat treatments applied to steels are related to transformation of the internal structure. The type, composition and metallographic structure of the transformation products have a substantial influence on the physical and mechanical peculiarities of steel. In other words; the physical and mechanical peculiarities of a steel depends on the type, amount and metallographic structure of the transformation products involved.
General Application of Heat Treatment
. Heating
. Keeping at Heated Temperature
. Cooling
We can group steels to undergo heat treatment in two distinct groups according to the amount of carbon they may involve;
1-Hypo-eutectoid steels (%C <0,8),
2-Hyper-eutectoid steels (%C > 0,8)
The heat treatment of steel is started with austenitization. Austenitization means heating of steel slowly up to a suitable temperature and its tempering (in other words until the internal structure shows similar structure in every region) until complete transformation of structure into austenite. For austenitization, steel material is heated up to a certain temperature over the lower critical temperature line (Ac1).
Hypo-eutectoid steels are subjected to austenitization process at temperatures 40-60 °C over the higher critical temperature line (Ac3). As to temperatures below Ac3 line, it causes soft spots in the steel, which prevents hardening of material.
As to hyper-eutectoid steels, they are austenitized at temperatures between Ac1 and the upper critical temperature line (Acm) pertaining to such steels. Since Acm line shows an immediate inc, very high temperatures are required to austenitize the entire structure.
The heated steel is kept at that temperature for a certain period of time in view of the type of the heat treatment, and is cooled at a certain cooling rate.
Issues to Consider in Applying Heat Treatment;
. The heating speed selected in heating of the steel up to the set temperature is less significant compared to other factors in heat treatment cycle. However, it is necessary to heat materials subjected to cold deformation to prevent distortion, in other words materials involving excessive internal stress should be heated slower than non-stress materials.
. Besides, the differences between the speed of heating or temperature rise in thin or thick sections should be heeded during heating of parts with varying sections. To reduce the distortion to occur in the part due to temperature, it is necessary to heat thin parts slower than thick parts. In general, steels are heated slowly to reduce the risk of damage during heat treatment.
. In increasing the steel temperature to those degrees where its internal structure transforms completely, undesired conditions may occur such as distortion, crack, oxidation, decarburization (separation of carbon atoms from internal structure) and grain growth. Therefore, steels are austenitized at temperatures as low as possible.
1-STRESS RELIEVING ANNEALING
Stress relieving annealing is the process of heating steel parts generally up to between 550-650 °C and then cooling them slowly to shape and to reduce internal tensions due to casting or welding procedures.
2-NORMALIZING
Normalizing is annealing over 40-60 °C (re-crystallization temperature) of the critical temperature of steel and then air cooling of steel materials for the purpose of making the crystal structure of the material more homogenous and thinner, and to distribute carbide properly in the next heat treatment.
3-QUENCHING
Cooling of steel heated up to a certain temperature (generally 850-1100 °C) in water, oil or salt baths to give it a martensite structure is called quenching. Cooling speed depends on the size of the part, hardenability of the steel and quenching environment. The most desired quenching speed is the slowest cooling speed good for providing the optimal hardness. If cooling speed is too high, cracks will occur in the part, and if too low, proper hardness cannot be obtained.
4-TEMPERING
Tempering is the process of eliminating brittleness generally by heating between 150-450 °C and cooling at a proper speed to relieve the tensions caused by cooling after quenching of steel hardened due to heat treatment, and to increase the steel's martensitic substance and resistance. Temper procedure should be realized immediately after quenching to reduce cracks to a minimum.
5-CEMENTATION
Cementation procedure is saturation of the surface of the low-carbon steel part with carbon. The procedure of carbon saturation is a result of gas-metal reaction by heating up to austenite phase temperature (850-950 °C) of the steel part in an environment containing carbon monoxide (CO).
Steel part is kept at cementation temperature for an adequate period of time to allow the carbon diffusion from the surface to the core advance as deep as desired. That period is called time of cementation. The advance depth of the carbon diffused from the surface of the steel part inwards within that period is called the cementation depth.
6-ANNEALING
The process of heating metal materials up to appropriate temperatures, keeping at that temperature until necessary adjustments are made, and slowly cooling thereafter for the purpose of acquiring desired structural, physical and mechanical peculiarities, and of facilitating removal of shavings and cold shaping is called annealing.
Soft Annealing
Soft annealing is reducing hardness by diminishing the grain size in the internal structure of steel, facilitating elimination of shavings or relieving internal tensions in casted and forged parts. Hypo-eutectoid steels are heated up to certain temperatures over Ac3 line, and hyper-eutectoid steels up to Ac1 lines, and they are kept within stove and cooled very slowly after transformation of their internal structure into austenite.
The transformations that occur in the internal structure of a coarse grain hypo-eutectoid steel part containing 0.5% C in the course of the annealing procedure are as follows;
a) the first or original structure from coarse ferrite and pearlite grains
b) Although pearlite turns into fine grain austenite at temperatures just above the Ac1 line, it remains the same in the ferrite structure. If we pass on to cooling from that temperature, then there would be no change in grain size because coarse ferrite grains were not changed
c) The structure turns entirely into fine grain austenite at temperatures just above the Ac3 line
d) When the part is cooled to room temperature, an internal structure is formed that contains fine ferrite grains and small pearlite areas
Thus, we infer that annealing at proper temperatures above Ac3 line is necessary for hypo-eutectoid steels to be subjected to soft annealing. For the hypo-eutectoid steels to be subjected to sound heat treatment, they should first have a homogeneous austenitic structure. To do so, it is recommended to anneal at the same temperature the steel materials heated up to austenitization temperature for a period of 1 hour per 25 mm wall thickness.
Hyper-eutectoid steels are subjected to austenitization process at temperatures approximately 50 °C over Ac3,1 line. Steels kept at those temperatures involve austenite and cementite phases. When steels are quenched at those temperatures, cementite parts remain in the structure exactly. It not only does not reduce cementite phase hardness but also increases the wear resistance of steels. Therefore, there is no need for total austenization of hyper-eutectoid steels. Those steels are annealed at a temperature at least 10 °C above the Ac3,1 line. The internal structures of hyper-eutectoid steels subjected to soft annealing consist of coarse lamella pearlite areas and surrounding extra-eutectoid cementite phase.The cementite network surrounding pearlite in that structure is hard and brittle. Presence of thick and hard grain borders in the internal structure hardens processing of steels via shavings method. Therefore, soft annealing is not applied as final treatment to hyper-eutectoid steels.
Normalization Annealing:
Normalization annealing is the process by which hypo-eutectoid steels are heated up to temperatures over approximately 40-50 °C of Ac3 and hyper-eutectoid steels of Acm transformation temperatures and then cooled at calm air after being annealed generally for the purpose of grain downsizing, obtaining a homogenous internal structure and mostly improving mechanical peculiarities.
The fundamental purposes of normalization annealing are;
a) grain downsizing,
b) obtaining a homogeneous internal structure,
c) distributing the carbide network which is in grain borders in hyper-eutectoid steels,
d) improving processing peculiarities of steels,
e) improving mechanical particularities, and
f) increasing the hardness and endurances of steels subjected to soft annealing
Therefore, normalization annealing can be the final heat treatment applied on steels.
It is a known fact that the cementite network formed in the structure of hyper-eutectoid steels subjected to soft annealing reduces the endurance of such steels. Normalization annealing ensures fragmentation and in some cases elimination to a great extent of cementite network in hyper-eutectoid steels. Therefore, the endurance of normalized steels show an increase.
A relatively high cooling is obtained in normalization annealing because the part is cooled in air. In general, the transformation temperature of austenite falls as the cooling speed rises, and finer pearlite is obtained.
Ferrite is a highly soft phase in contrast to cementite, which is very hard. Hardness of steel increases because of the close or frequent alignment of cementite layers in the normalized steel's structure. Therefore, the hardness and endurance of normalized steels are significantly higher compared to the values in question of steels subjected to soft annealing. Table 1 provides the mechanical peculiarities of some steels when subjected to soft annealing and normalized.
Spheroidizing Annealing:
Spheroidizing annealing is the process by which carbides are spheroidized through slow cooling after steels are kept for a long time around Ac1 temperature line, and then annealed by oscillation at that zone. The process can also be made through controlled cooling after austenitization. As specified in the soft annealing process, hyper-eutectoid steels in annealed form are not suitable for processing because they have hard and brittle cementite grains in their internal structure. Spheroidization annealing is used to facilitate processing of such steels and to increase ductility.
Spheroidization annealing is realized through one of the following methods.
a) Steel material is annealed for a long time (15-25 hours) at a temperature immediately below Ac1 line (for example 700 °C).
b) Steel material is heated and cooled between temperature immediately below and above the critical low temperature line (Ac1), in other words, it is annealed by oscillation.
c) After the material is annealed at a temperature above the Ac1 critical temperature line, it is either cooled very slowly in the stove or kept for a long time at a temperature immediately below Ac1 line.
The annealing procedure at high temperature causes fragmentation and dissolution of the pearlitic structure and the cementite network inside the steel. As a result of the spheroidization annealing, a ferritic matris and an internal structure consisting of spheroid carbides distributed within are obtained. As a result of the spheroidization annealing, the hardness of steel diminishes as its ductility increases. Hyper-eutectoid steels become available for processing as a result of that process.
Spherodization annealing is applied mostly to high carbon steels. Low carbon steels are rarely subjected to spheroidization annealing. Because; such steels highly soften as a result of the spheroidization steels, and such excessive softening causes some difficulties in the course of the shavings process. Medium carbon steels are sometimes subjected to spheroidization annealing before plastic shaping process to give them adequate ductility. It is necessary to adjust annealing period well in the course of spheroidization annealing. If steel is annealed for a period longer than necessary, then cementite particles merge and augment, which adversely affects the processing capacity of the steel.
Softening, spheroidization and normalization processes are applied to make steels available for processing. However, the heat treatment to apply is selected in view of the steel's carbon rate.
Stress Relieving Annealing and Intermediate Annealing
Stress relieving annealing is the process of heating up to a suitable temperature below transformation temperatures and then slowly cooling of metallic materials for the purpose of mitigating internal tensions caused by casting, welding and cold shaping processes. The process is sometimes also called transformation temperature or below critical temperature annealing. Steel materials are subjected to tension relieving annealing between temperatures of 540°C and 630°C.
Intermediate annealing is a process very much similar to tension relieving annealing, which comprises of slow cooling after recrystallization after heating of steel materials up to a temperature immediately below Ac1 transformation temperature (550-680 °C) to continue cold shaping in plate and wire construction from hypo-eutectoid steels.
Quenching Hardening
As steels are cooled at a slow or medium speed after the annealing process, carbon atoms dissolved within the austenite are separated from austenite structure via diffusion. When cooling speed is increased, carbon atoms are unable to find adequate time to leave solid dissolution via diffusion. Even if iron atoms move to some extent, a different structure forms due to imprisonment of carbon atoms within the solution. That structure comprising of rapid cooling is called "martensite".
The most important reason why martensite hardness is high is the fact that the lattice structure is distorted. Some volumetric growth occurs in steel materials in the course of martensite transformation. The volumetric growth in question causes local tensions at very high levels resulting in excessive distortion or plastic deformation of the structure of steels. Distortion of the lattice structure increases the hardness and endurance of the quenched steels.
The martensite formed after the quenching process appears like a pin or prick under the microscope, and sometimes exhibits an appearance that looks like a truss. Martensitic structure is vague and pale in most of the steels, and therefore cannot be distinguished easily. Because high carbon steels, in contrast, form a residual austenite background, the structure of martensite in the form of pin or prick gains a more evident appearance.
Martensitic transformation occurs only in the course of cooling. Therefore, the transformation in question is independent of time, in other words, depends on lower temperatures, that is, cooling. The most significant peculiarity of martensite is that it is a very hard phase. The hardest phase after cementite is martensite in steels. High hardness values are obtained in steels involving an adequate amount of carbon.
It can be said that the highest hardness that can be acquired from unalloyed quenched steel (in martensitic condition) depends only on the steel's carbon ratio. The reason why martensite hardness is high is because the lattice structure is distorted due to excessive saturation resulting from the fact that it involves a higher rate of carbon compared to the ratio soluble with martensite solid dissolution.
The fundamental purpose of the hardening process is only to acquire a martensitic structure. To do so, its cooling at speeds higher than a value called critical cooling after the annealing process of the material
Quenching Media
The ideal quenching medium should ensure that the cooling speed in the beginning is high, and that the cooling speed at low temperatures is low to prevent distortion of the material. However, there is no quenching medium to fully ensure that case. The quenching liquids such as water and aqueous solutions of inorganic salts ensure that cooling speeds in the beginning are high. However, distortion and crack may occur in the material since the cooling speeds continue at low temperatures. A long circuit A is obtained with traditional quenching oils and a short circuit B with low cooling speed.
The quenching media used in the industry are listed as follows in view of their quenching intensities.
a) Salty water
b) Tap water
c) Molten or liquid salts
d) Oil and water mixture
e) Oil
f) Air
Tempering
Because the martensitic structure acquired via quenching process in steels is brittle, it is not suitable for several applications. Besides, martensite formation causes formation of internal tensions inside the steel. Therefore, the annealing process applied to quenched steels at temperatures almost always below Ac1 line is called tempering. The purpose of tempering is to eliminate the residual tension of the quenched steel and to increase the ductility and saturation of the steel. When quenched steels are tempered, their ductilities increase, yet their hardness and endurance diminish.
Generally, hardness falls and saturation increases as tempering temperature (200ºC to 425ºC) increases. As the tempering temperature increases, the hardness and endurance values of the quenched steels decrease, in contrast to ductility and saturation values, which increase.
The steel parts used in applications that require high hardness and high wear resistance are tempered at temperatures below 205 °C, and those used in applications requiring high saturation at temperatures above 425 °C. If the part bears no notch to cause aggregation of stress, change of ductility can be considered as a good measure for saturation, in which case tempering process may not be inconvenient at temperatures between 205 to 425 °C. When the tempering temperature reaches the value 205ºC, residual tensions can be eliminated to a great extent. At 480ºC, residual tensions are completely eliminated.
Since tempering is a power-related process, both temperature and period are significant parameters that affect the tempering process. The same tempering effects can be acquired in a short time at high temperatures or in a long time at low temperatures. Figure 14 shows the effect of tempering period on the hardness of steel in eutectoid composition tempered at different temperatures.
Martempering
After the part to be hardened is subjected to austenitization process, it is soaked into a lead or salt bath kept at a temperature immediately above the beginning temperature (Ms) of martensitic transformation. The part is kept inside the bath until the temperatures of the surface and the center are the same, in other words, the same temperature is acquired along the entire section. Then the part is quenched to gain a totally martensitic internal structure. With the process, shrinking incident caused by cooling is separated from the expansion due to austenite-martensite transformation both to prevent quenching crack in big parts and to harden the part.
Austempering
After the part to be hardened is austenitized, it is soaked into a lead or salt bath kept at a temperature above the beginning temperature (Ms) of martensitic transformation. The part is kept in bath until transformation is complete, and then air cooled having been taken from the bath afterwards.


 Copyright © NSC Çelik Dýþ Tic.Paz.San. ve Tic.Ltd.Þti. - 2014 All rights reserved.
Copyright © NSC Çelik Dýþ Tic.Paz.San. ve Tic.Ltd.Þti. - 2014 All rights reserved.